Michael Snyderman, MD
In the 1970’s three independent laboratories reported the presence of a MLRV in sarcoma, leukemia and various lymphomas [1-6]. The MLRV was found only in neoplastic and not in normal cells and was similar to the Rauscher virus. The most recent nomenclature calls this type of virus an HGRV. Surprisingly, the 1970’s body of work was largely ignored for the next thirty years until 2006 when Urisman et al [7] reported a HGRV/MLRV which they named XMRV in some prostate cancer. In 2009, Lombardi et al [8] at the Whittemore Peterson Institute (WPI) found evidence of infection with HGRV/MLRVs similar to XMRV in most patients with the Chronic Fatigue Syndrome (CFS). At present, the Lombardi et al viral findings have been criticized because many laboratories have not been able to reproduce their results. Some retrovirologists have said unequivocally that HGRV/MLRVs do not cause CFS.
The diagnosis of CFS is objectified by using the Canadian Criteria for diagnosis of CFS and by demonstrating a typical elevation of cytokines and chemokines [9]. CFS patients are suspected to have an increased incidence of lymphoid malignancy and brain tumors compared to the normal population [10]. The implication of this is that HGRV/MLRVs may be etiological for both CFS and malignancy. Daniel Peterson’s CFS practice consisted of 300 patients from the 1984 Nevada CFS epidemic. Thirteen patients from this cohort developed various B-cell lymphoproliferative disorders and all that were tested were positive for a T-cell clonal expansion. They were also found to have evidence of infection with HGRV/MLRV at the WPI but this latter data is now in limbo. It was hypothesized that a HGRV/MLRV infection was responsible for the T-cell clonal expansion and that this clonal T-cell expansion might have promoted the development of CFS and malignancy.
Treatment of MLRV associated malignancy has not previously been reported. The retrovirus HTLV-1 associated T-cell lymphoma/leukemia does respond to AZT and IFNa [11, 12]. In addition, multiple human tumor cell lines including breast and ovarian cancers show growth inhibition and apoptosis when exposed to AZT [13, 14]. Several groups reported inhibition of XMRV by FDA approved antiretrovirals including AZT, raltegravir and tenofovir in cell culture [15, 16]. The response of a patient with CFS and a MLRV associated malignancy to anti-retroviral drug therapy would support a role of MLRV in both. We had the opportunity to study such a patient with both CFS and CLL who was positive for evidence of infection with MLRV and was also positive for a T-cell clonal expansion. He had cytokine and chemokine elevations consistent with the diagnosis of CFS. This patient was a Medical Oncologist who was well acquainted with the therapeutic options and guidelines for both disorders and decided to undergo anti-
retroviral therapy.
Results
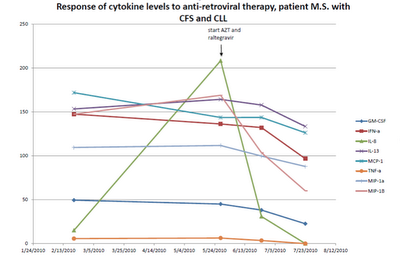
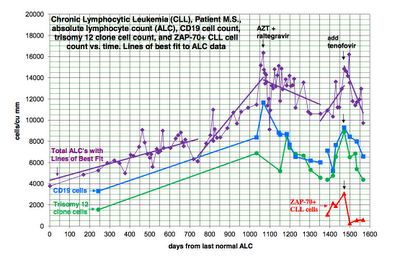
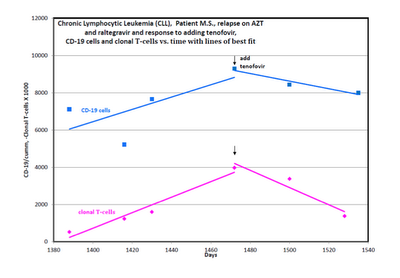
A patient with B-cell CLL tested positive for antibodies to MLRV proteins. Integration studies are pending. Although not previously diagnosed as having CFS, his symptoms fulfilled the Canadian Criteria for diagnosis of CFS. His elevated cytokine and chemokine levels were consistent with the diagnosis of CFS. He was also positive for a clonal T-cell expansion by both quantitative and qualitative assays for the presence of a clonal TCRg gene rearrangement. He had average prognostic factors with the only potential adverse factor being a trisomy 12 clone. His testing for EBV, CMV, HHV6A and HHV6B was negative. He started treatment with AZT and raltegravir 571 days after diagnosis of CLL. By day 56 of treatment, his cytokine levels had improved (Figure 1). This coincided with improvement in symptoms of CFS which included fatigue, difficulty in concentration and neuropathic pain. His response continued for 9.4 months with no associated toxicity and he was able to work full time. His previously increasing absolute lymphocyte count (ALC), CD 19 cells and trisomy 12 cells trended downward during this period of time. At the start of treatment with AZT and raltegravir, his ALC was 16,348/cu mm and CD 19 cells 11,658/cu mm up from 3,303 at diagnosis. His trisomy 12 cells peaked at 8,490/cu mm day 117 of treatment up from 1,550 at diagnosis. After 285 days of treatment his ALC was down to 10,600/cu mm, CD 19 cells down to 6,015 and trisomy 12 down to 4,558. Subsequently, his counts relapsed despite continuation of AZT and raltegravir. Symptoms of CFS also worsened. Tenofovir was added and all parameters trended down again and symptoms of CFS improved (Figure 2). The ALC peaked at 16,194 at week 3 of treatment and by week 20 was down to 12,324. The baseline CD-19 count was 9,298, trisomy 12 cell count was 8,902 and the ZAP70 count was 3,068. By week 13 the CD-19 count was down to 6,570, the trisomy 12 count was down to 4,374 and the ZAP70 count was down to 591. Week 20 values of these last three parameters are pending. Quantitative data for clonal T-cells became available at the time of relapse and showed a rise in clonal T-cells that appeared to be more rapid than the increase in the CD-19 cells and after the addition of tenofovir both the clonal T-cells and the CD-19 cells trended down but the clonal T-cells appeared to decrease more rapidly (Figure 3).
Discussion
The development of B-cell lymphoid malignancies in 13 of 300 CFS patients suggests that CFS patients are at a several hundred fold increased risk for malignancy compared to generally quoted incidences for the general population. Of these 13 patients, all were positive for a clonal T-cell expansion. They were also positive for evidence of infection with HGRV/MLRVs but this data is now in question.
The greatly increased risk for B-cell malignancy in a potentially HGRV/MLRV infected population may be due to infection of the B-cell line by the HGRV/MLRVs. Retroviruses have been thought to cause cancer by insertional mutagenesis. This mechanism requires that the retrovirus proviral DNA be integrated into host cell DNA next to a proto-oncogene thereby inducing activation of the proto-oncogene. A more important mechanism with MLRVs may be the ability of viral proteins to change host cell gene expression. Twenty-four to forty-eight hours after a permissive cell line is infected with XMRV, multiple cellular genes are expressed: “10 genes are implicated in cell morphology, 11 genes in cellular development, 12 genes in cell-to-cell signaling and interaction, 11 genes in cellular movement and 13 genes in cellular growth and proliferation” [17]. Spadafaro has shown that reverse transcriptase can cause gene activation and lead to the malignant phenotype [18]. In some retrovirus related cancers, Env [19] and Gag [20] may also be important in malignant transformation.
The finding that XMRV did not cause malignant transformation de novo in tissue culture [21] would be irrelevant to the clinical reality of human cancer. It is accepted that multiple events are necessary to convert a cell line into a pre-neoplasm or a clinically important neoplasm. Human cancers have mutated genes and changes in gene expression that could make them permissive to infection by retroviruses. The retroviruses could induce further changes in gene expression that would make the infected cell line behave in a more malignant fashion. The corollary to this is that treatment that would block viral protein influence in a neoplastic cell line could make the neoplastic cell behave in a less malignant way.
A complementary hypothesis is that infection by HGRV/MLRVs results in a T-cell clonal expansion. The clonal T-cells produce elevated cytokine and chemokine levels which may be partially responsible for the CFS. Furthermore these cytokines and chemokines may have a paracrine activity that would stimulate a simultaneous neoplasm to behave in a more aggressive fashion [22].
One objection to considering HGRV/MLRVs to be pathogenic viruses is that there was previously no explanation as to how MLRVs could have entered the human population. However, early vaccines were prepared by passaging human virus through mice for the purposes of viral isolation and for attenuation. This would have allowed for contamination of vaccines with murine leukemia viruses [23]. The original Yellow Fever Vaccine was made in the early 1930’s by culturing the virus in mouse cerebral tissue [24]. Some patients received both the Yellow Fever virus and infected mouse cerebral tissue. The YF17D strain was used to immunize over 400 million people world-wide over the next 65 years [25]. The U.S. Armed Forces started to vaccinate service men for Yellow Fever during WWII and continued thereafter as per the branch of service and deployment status [26]. The polio vaccination trials in the United States started in 1952. The polio virus strains were initially serially transferred by Koprowski through many passages in mice, cotton rats and primates to achieve attenuation [27]. Olitsky with whom Sabin had a long-term collaboration adapted the type 2 (Lansing) polio strain to mice [28] and the Sabin vaccine contained this strain. Indeed, the patient studied here, received the live oral polio vaccine in the early 1960s, ten years later developed symptoms of CFS and forty years later developed CLL. He also received the Yellow Fever vaccine in the early 1970’s on entering the Armed Services.
In summary, a new patient with both CFS and B-cell CLL was identified. Infection with a HGRV/MLRV was suggested by the presence of antibodies to MLRV proteins. He also was positive for a T-cell clonal expansion and had elevated cytokine and chemokine levels typical of patients with CFS. With anti-retroviral therapy he showed improvement in his cytokine and chemokine levels, CFS symptoms and hematological parameters. Presumably his improvement was related to the anti-retroviral effects of treatment. The progressive improvement of his ALC, CD19 cells and trisomy 12 clone lasted 9.4 months. Despite continuation of AZT and raltegravir his leukemia relapsed. Interestingly, during relapse both the total B-cell count and the clonal T-cells increased with the rate of increase of the T-cells appearing more rapid. A second response was induced by the addition of the second reverse transcriptase inhibitor, tenofovir. Both the total lymphocyte count and the clonal T-cells fell with the rate of decrease of the clonal t-cells appearing more rapidly. At the time of this report the second response is ongoing at 14-20 weeks.
These findings are consistent with the importance of reverse transcriptase in the behavior of the patient’s leukemia and CFS and the potential influence of the clonal T-cells on both these processes. It is possible that inhibiting reverse transcriptase decreased proliferation of both the T and B-cell clones or the effect might be primarily on the clonal T-cells. The rise and fall of cytokines we have documented would be proportional to the absolute number of the clonal T-cells and secondarily could influence the proliferation of the B-cell clone.
Alternative explanations for the therapeutic effect of his anti-retroviral therapy have been considered. One of these is selective toxicity rather than an anti-retroviral effect. Selective toxicity has never before been seen with the hundreds of agents used as cancer therapeutics and seems an unlikely explanation for his improvement especially as the patient had no toxicity at all. AZT, raltegravir and tenofovir have never been shown to have single agent chemotherapy activity so a chemotherapy effect is unlikely to be an explanation. Anti-telomerase activity of the AZT has also been considered, but the rapid response to treatment does not fit the kinetics of depletion of telomeres and induction of apoptosis. Furthermore, an anti-telomerase agent did reach clinical trial and failed to induce remissions. Anti-herpesvirus activity of the antiretroviral regimen is not a tenable explanation of his response as an active herpesvirus infection was ruled out.
There is nothing unique about this patient’s clinical presentation to suggest that his case is any way unrepresentative. His response to anti-retroviral therapy implies that HGRV/MLRVs were etiological for both his CFS and CLL and that anti-retroviral therapy might help other patients with CFS and HGRV/MLRV associated malignancy. Many more patients need to be studied. Ultimately questions that should be answered are what neoplasms are associated with HGRV/MLRVs, will existing anti-retroviral drugs have activity in these neoplasms, will other anti-retroviral drugs such as a protease inhibitor be required and what would be the optimal combination of anti-retroviral drugs.
- Kufe D, Hehlmann R, Spiegelman S: Human sarcomas contain RNA related to the RNA of a mouse leukemia virus. Science 1971, 175:182-185.
- Hehlmann R, Kufe D, Spiegelman S: RNA in human leukemic cells related to the RNA of a mouse leukemia virus. Proc. Nat. Acad. Sci. USA 1972, 69:435-439.
- Hehlmann R, Kufe D, Spiegelman S: Viral-related RNA in Hodgkins’ Disease and other human lymphomas. Proc. Nat. Acad. Sci. USA 1972, 69:1727-1731.
- Kufe D, McGrath IT, Ziegler JL, Spiegelman S: Burkitt’s tumors contain particles encapsulating RNA-instructed DNA polymerase and high molecular weight virus related RNA. Proc. Nat. Acad. Sci. USA 1973, 70:1737-741.
- Baxt WG: Sequences present in both human leukemic cell nuclear DNA and Rauscher Leukemia Virus. Proc. Nat. Acad. Sci. USA 1974, 71:2853-2857.
- Aulakh GS, Gallo RC: Rauscher-leukemia-virus-related sequences in human DNA: Presence in some tissues of some patients with hematopoietic neoplasias and absence in DNA from other tissues. Proc. Nati. Acad. Sci. USA 1977, 74:353-357.
- Urisman A, Molinaro RJ, Fischer N, Plummer SJ, Casey G, Klein EA, Malathi, K, Magi-Galluzzi C, Tubbs RR, Ganem D, Silverman RH, DeRisi JL: Identification of a novel gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog 2006, 2:e25.
- Lombardi VC, Ruscetti FW, Das Gupta J, Pfost MA, Hagen KS, Peterson DL, Ruscetti SK, Bagni RK, Petrow-Sadowski C, Gold B, Dean M, Silverman RH, Mikovits JA: Detection of an infectious retrovirus, XMRV, in blood cells of patients with Chronic Fatigue Syndrome. Science 2009, 585-589.
- Lombardi VC, Hagen KS, Hunter KW, Diamond JW, Smith-Gagen J, Yang W, Mikovits JA: Xenotropic Murine Leukemia Virus-related Virus-associated Chronic Fatigue Syndrome reveals a distinct inflammatory signature. In vivo 2011, 25: 307-314.
- Levine PH, Fears T R, Cummings P, Hoover RN: Cancer and a fatiguing illness in Northern Nevada-A causal hypothesis. AEP 1998, 8:245-249.
- Matutes E, Taylor GP, Cavenagh J, Pagliuca A, Bareford D, Domingo A, Hamblin M, Kelsey S, Mir N, Reilly JT: Interferon a and zidovudine therapy in adult T-cell leukaemia lymphoma: response and outcome in 15 patients. British J Hematology 2001, 113:779-784.
- Bazarbachi A, Ghez D, Lepelletier Y, Nasr R, de The H, El-Sabban ME, Hermine O: New therapeutic approaches for adult T-cell leukaemia. The Lancet Oncology 2004, 5:664-672.
- Melana SM, Holland JF, Pogo B G-T: Inhibition of cell growth and telomerase activity of breast cancer cells in vitro by 3’-Azido-3”-deoxythmidine. Clinical Cancer Research 1998, 4:693-696.
- Li H, Song T, Xu W, Yu Y, Xin X, Hu D: Effect of 3’-azido-3’-deoxythymidine (AZT) on telomerase activity and proliferation of HO-8910 cell line of ovarian cancer. Int J Biomed Sci 2005, 2:35-41.
- Singh IR, Gorzynski JE, Drobysheva D, Bassit L, Schinazi RF: Raltegravir is a potent inhibitor of XMRV, a virus implicated in prostate cancer and Chronic Fatigue Syndrome. PLoS Pathog 2010, 5:e9948.
- Paprotka T, Venkatachari NJ, Chaipan C, Burdick R, Delviks-Frankenberry KA, Hu W-S, Pathak VK: Inhibition of Xenotropic Murine Leukemia Virus-Related virus by APOBEC3 proteins and antiviral drugs. J of Virology 2010, 84:5719-5729.
- Lee M, Gusho E, Das Gupta J, Klein E, Silverman R: XMRV infection induces host genes that regulate inflammation and cellular physiology [abstract 280]. J Urology 2011, 185(suppl 4):e 113.
- Sciamanna I, Landriscina M, Pittoggi C, Quirino M, Mearelli C, Beraldi R, Mattei E, Serafino A, Cassano A, Sinibaldi-Vallebona P, Garaci E, Barone C, Spadafora C: Inhibition of endogenous reverse transcriptase antagonizes human tumor growth. Oncogene 2005, 24:3923–3931.
- Katz E, Lareef MH, Rassa JC, Grande SM, King LB, Russo J, Ross SR,MonJG: MMTV env encodes an ITAM responsible for transformation of mammary epithelial cells in three-dimensional culture. JEM 2005, 201:431-439.
- Swanson I, Jude BJ, Zhang AR, Pucker A, Smith ZE, Golovkina TV: Sequences within the gag gene of mouse mammary tumor virus needed for mammary gland cell transformation. J Virology 2006, 80:3215–3224.
- Metzger MJ, Holguin CJ, Mendoza R, Miller AD: The prostate cancer-associated human retrovirus XMRV lacks direct transforming activity but can induce low rates of transformation in cultured cells. J Virology 2010, 84: 1874-1880.
- Erdman S., Poutahidis T: Roles for Inflammation and Regulatory T Cells in Colon Cancer. Toxicologic Pathology, 2010, 38: 76-87, 2010.
- van der Kuyl AC, Cornelissen M, Berkhout B: Of mice and men: on the origin of XMRV. Frontiers in Microbiology 2011, 1:1-7.
- Theiler M: Nobel Lecture, December 11, 1951. In Nobel Lectures, Physiology or Medicine 1942-1962, Elsevier Publishing Company; 1964: I:yellow fever Max Theiler – Nobel Lecture.mht
- Rockefeller University: Yellow Fever immunization statistics. In ScienceDaily www.sciencedaily.com/releases/2010/06/100611222839.htm
- Millitary vaccinations. Air Force Joint Instruction 48-110, Army Regulation 40-52, BUMEDINST 6230.15, CGCOMTINST M6230.4E, dated 12 May 2004.
- Koprowski H: Historical aspects of the development of live virus vaccine in poliomyelitis. Brit Med J 1960, 2:85-91.
- Casals J, Olitsky PK, Anslow RO: Adaption of a Lansing strain of poliomyelitis virus to newborn mice. JEM 1951, 94:111-121.

>There's a diagram, right at the end of this page http://davidsbell.com/ and it got me wondering if CFS is like a siege state?
Imagine your body is a castle with defences. Some bad guys try to invade (infection).
Suppose you don't see them coming? The life and business of the castle continue as normal right up until the bad guys have got in, and then – bad guys everywhere!
Suppose you do see them coming? You try to stop them getting in, but they just keep coming. Soon you are using every resource you have to keep them out – the normal life and business of the castle is suspended, but the invaders haven't actually got inside.
The first situation would be like feeling totally fine while the damage was done, and you wouldn't know until you had cancer.
The second situation would be like having ME, you have seen what's happening, and you are in the fight of your life, but because no invaders are seen within the castle walls, your doctor declares no problem here, even though it's taking everything you've got to keep them out.
K
>I think I love you, Dr. Snyderman! Thank you so much for sharing.
I recently uncelebrated my 27th anniversary with this lovely disease, which I got at 17. So, a question:
My Dad's side of the family has an "inherited leukemia." I don't recall the name, but it hits family members in their 40's and they die very, very quickly. On his side of the family, if you make it out of your 40's, you're golden.
I'm 44.
I also have a toddler (it is very hard but she makes my life worth living) with Down syndrome. As I'm sure you're aware, kids with Ds have an elevated risk of getting leukemia.
I'm asking you a question I can't even formulate. Could she be at even a higher risk? What the h*ll do I do if she gets it and ARV's could save her life but chemotherapy will be pushed on her?
Dr. Jamie has my email if this is too complicated to write about it here.
Thanks,
AlphaHusky
>Don't you think a study like this needs to be more than n=1?
Seriously, how do you expect to publish this? You don't even error bars, standard devs, conf. intervals… etc… etc…