What’s occurred in the last 30 years is criminal, Mikovits says today. “Mothers and fathers got sick, their children got sick.” But with heightened attention, she adds, patients are likely to get help soon. Even lacking a causal pathogen, biomarkers in this patient population can be studied for clues. “We can find therapies for the CFS patient population even before we determine the exact cause,” Mikovits says.Chasing the Shadow Virus by Hillary Johnson Discover March 2013.
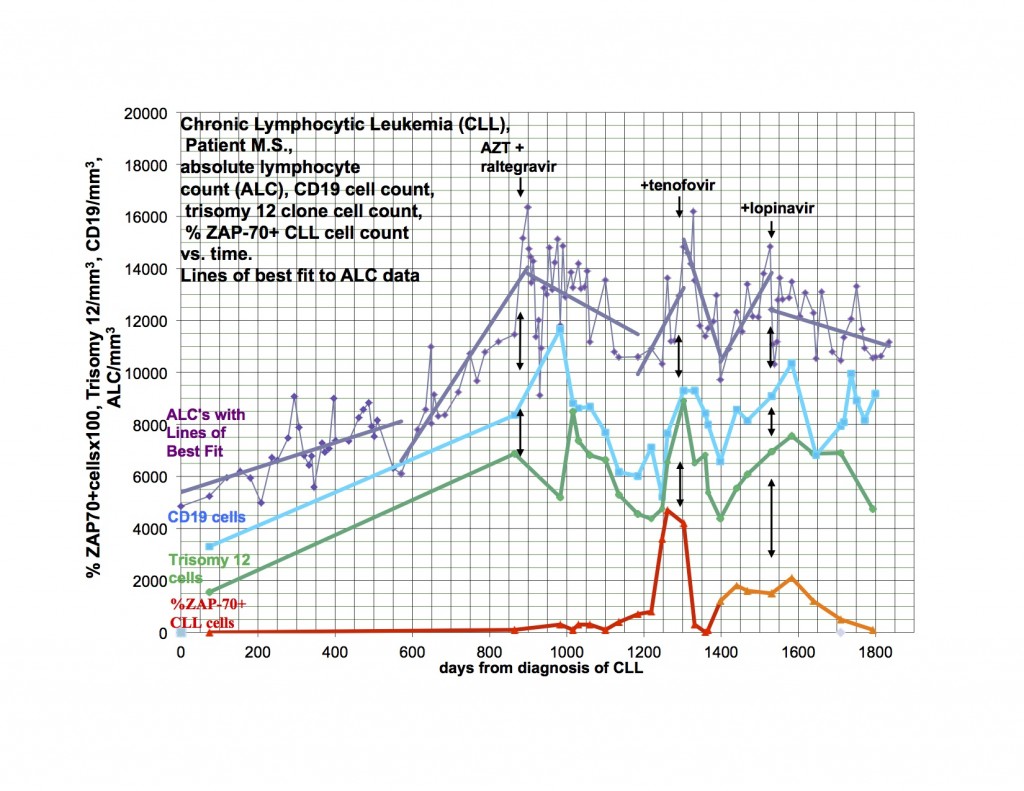
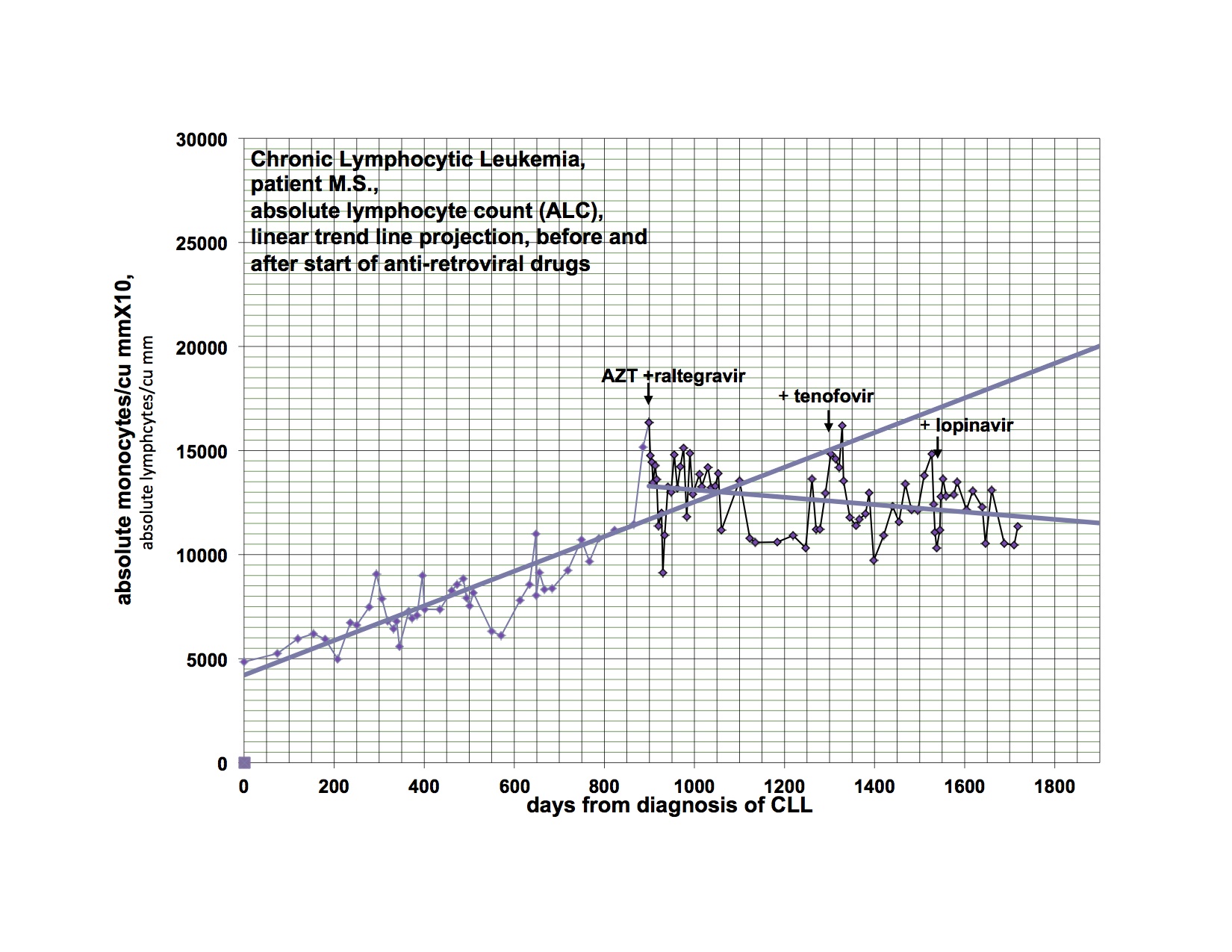
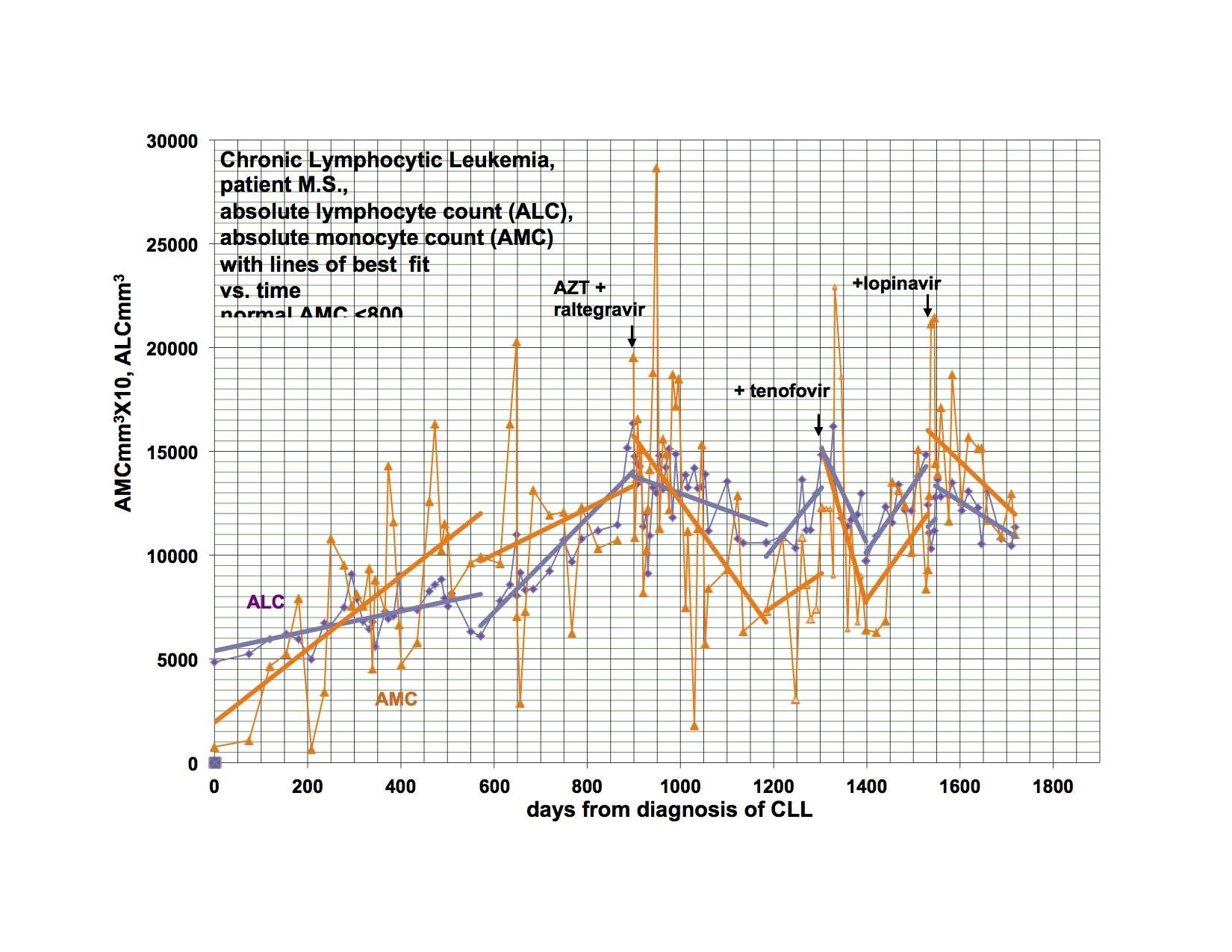
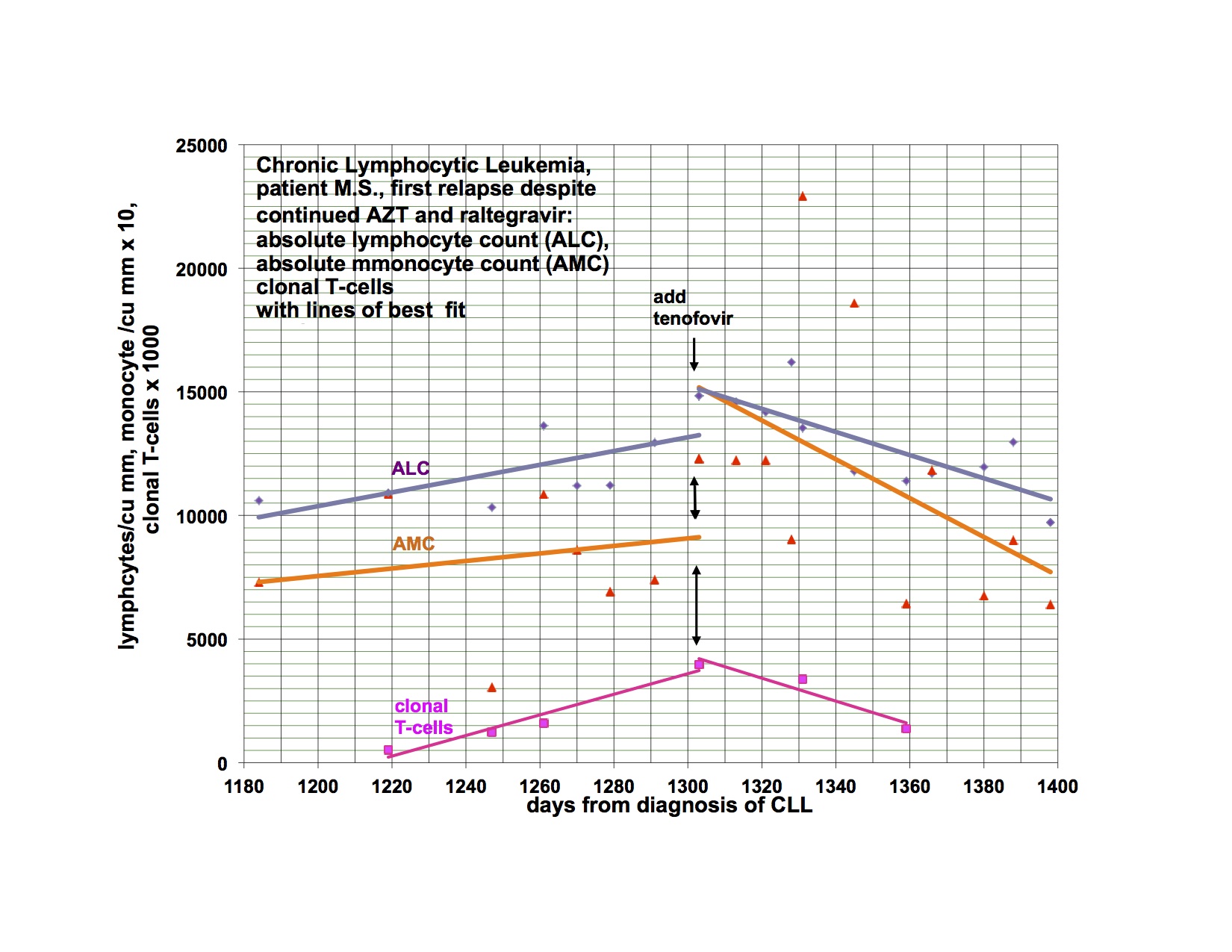
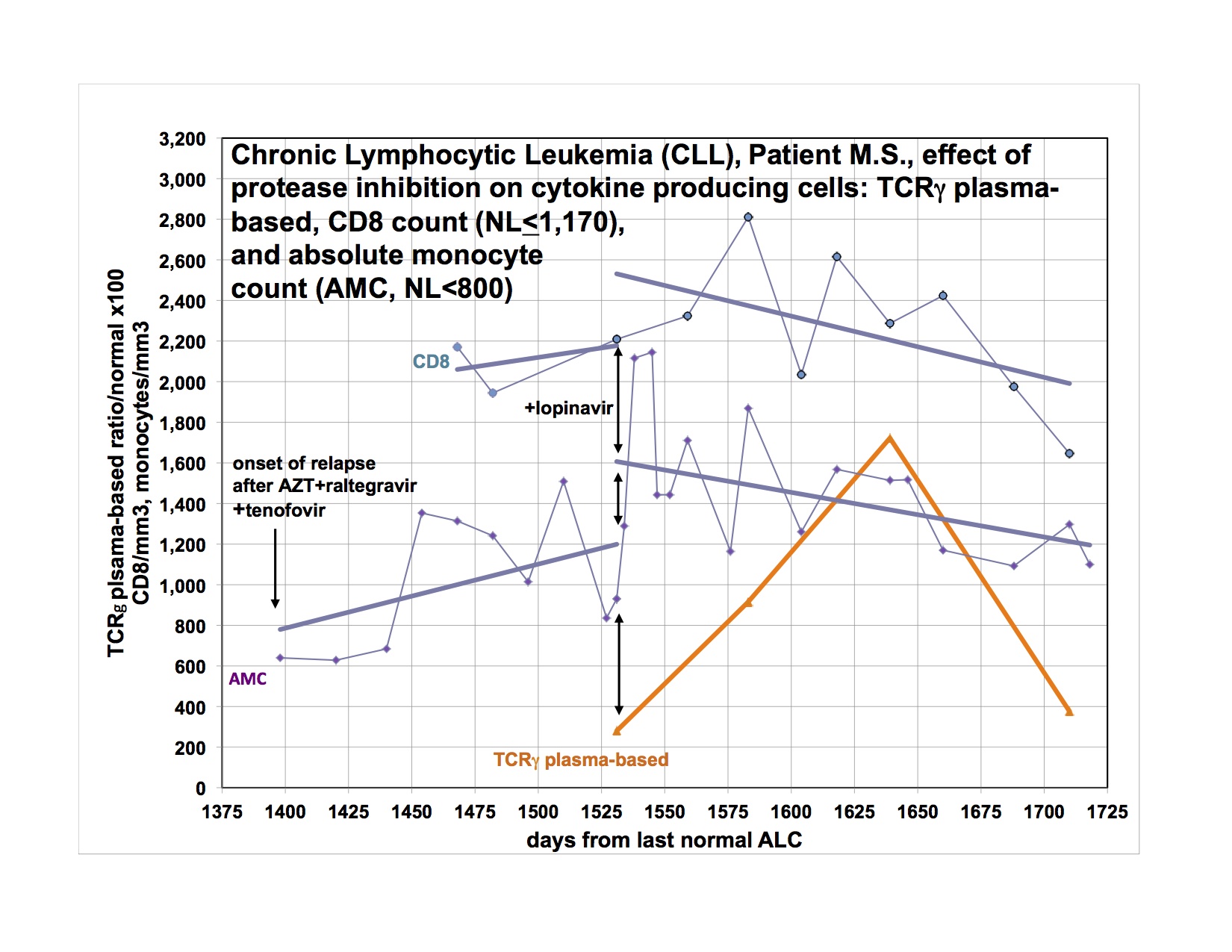
As I said last time, I started Viread again, because I became dangerously hypertensive, a few weeks after stopping it. I had a significant drop in my BP, almost to normal from days 6-12, then it went up again, not quite as high as before, but very high. After much fiddling, it is now controlled, but I had to add additional antihypertensive medication. Happily, after a month back on Viread, there is a downward trend again and I’m hoping I’ll be able to wean from the extra treatment soon. This is not the first time I’ve had this problem, but it was the worst episode yet, and was related in time to stopping Viread. I have been feeling significantly better for the last week, and am also back to baseline productivity. I flared for the first few weeks I went on Viread the first time also. I am going to Tucson to see patients in a couple of weeks and when I come home, am planning to restart Isentress and then Kaletra. I really didn’t want to go back on Viread, but it does seem that I’m getting a payoff again from it. I went off because I wasn’t doing well, and things got even worse, now better back on. I am just reporting, not explaining why or how. The disease is a relapsing remitting illness all on it’s own and changes may or may not have anything to do with the last thing you did.
My reading lately has been about retrotransposons and HERVs, especially MSRV, multiple sclerosis-associated retrovirus. Here is a cutting edge, must read paper, senior author Hervé Perron, whose name appears on most of the important papers on this topic: The DNA Copy Number of Human Endogenous Retrovirus-W (MSRV-Type) Is Increased in Multiple Sclerosis Patients and Is Influenced by Gender and Disease Severity.
MSRV increases its copy number in PBMC of MS patients and particularly in women with high clinical scores. This may explain causes underlying the higher prevalence of MS in women. The association with the clinical severity calls for further investigations on MSRV load in PBMCs as a biomarker for MS.
The envelope protein from multiple sclerosis (MS) associated retroviral element (MSRV), a member of the Human Endogenous Retroviral family ‘W’ (HERV-W), induces dysimmunity and inflammation.
Env antigen was detected in a serum of 73% of patients with MS with similar prevalence in all clinical forms, and not in chronic infection, systemic lupus, most other neurological diseases and healthy donors (p<0.01). Cases with chronic inflammatory demyelinating polyneuropathy (5/8) and rare HC (4/103) were positive. RNA expression in PBMC and DNA copy numbers were significantly elevated in patients with MS versus HC (p<0.001). In patients with MS, DNA copy numbers were significantly increased in chronic progressive MS (secondary progressive MS vs relapsing-remitting MS (RRMS) p<0.001; primary progressive MS vs RRMS -<0.02). Env protein was evidenced in macrophages within MS brain lesions with particular concentrations around vascular elements.
The above paper concludes that exogenous virus production is unlikely. Particles have been identified in MS patients going back to 1989: Leptomeningeal cell line from multiple sclerosis with reverse transcriptase activity and viral particles.
In fact, a virus was identified in MS in 1975. Look at how far they got with the technology at hand at that time: Multiple sclerosis-associated agent: transmission to animals and some properties of the agent.
In confirmation and extension of observations by Carp and his associates, brain tissue and sera from patients with multiple sclerosis (MS) were found to harbor an agent which induces a transitory depression in polymorphonuclear leukocytes (PMN) in mice as well as in rats, hamsters, and guinea pigs. All of eight MD brains contained this agent at titers as high as 10(-9)/g of brain tissue. The agent was found in MS sera at titers up to 10(-3)/ml of serum, but its presence depended to some extent on the clinical status of the patients; it was observed more frequently in sera of patients with active disease (73%) thatn in sera of patients with quiescent disease (31%). Control brain tissues or sera failed to induce PMN depression. The apparently MS-associated agent (MSAA) passed through 50-nm but not 25-nm membrane filters (Millipore Corp.) and was largely sedimented at 105,000 X g but not at 50,000 X g for 1 h. It multiplied to high titers in the central nervous tissue of the inoculated animals and could be serially transmitted from animal to animal by passage of brain homeganates. Various observations and considerations appear to preclude that MS-associated agent represents an indigenous animal virus. Although its role in MS remains to be determined, it should be considered a candidate for the etiology of this disease.
Endogenous retroviral genes, Herpesviruses and gender in Multiple Sclerosis contains electron micrographs of MSRV particles.
Endogenous retroviruses represent about 8% of the human genome and belong to the superfamily of transposable and retrotransposable genetic elements. Altogether, these mobile genetic elements and their numerous inactivated “junk” sequences represent nearly one half of the human DNA. Nonetheless, a significant part of this “non-conventional” genome has retained potential activity. Epigenetic control is notably involved in silencing most of these genetic elements but certain environmental factors such as viruses are known to dysregulate their expression in susceptible cells. More particularly, embryonal cells with limited gene methylation are most susceptible to uncontrolled activation of these mobile genetic elements by, e.g., viral infections. In particular, certain viruses transactivate promoters from endogenous retroviral family type W (HERV-W). HERV-W RNA was first isolated in circulating viral particles (Multiple Sclerosis-associated RetroViral element, MSRV) that have been associated with the evolution and prognosis of multiple sclerosis. HERV-W elements encode a powerful immunopathogenic envelope protein (ENV) that activates a pro-inflammatory and autoimmune cascade through interaction with Toll-like receptor 4 on immune cells. This ENV protein has repeatedly been detected in MS brain lesions and may be involved in other diseases. Epigenetic factors controlling HERV-W ENV protein expression then reveal critical. This review addresses the gene-environment epigenetic interface of such HERV-W elements and its potential involvement in disease.
Here is a paper about something that could turn into useful therapy, overlooking the significant risks associated with the administration of monoclonal antibodies and the inherent risks involved in hybridoma technology, which involves fusing human cancer with animal B cells. GNbAC1, a humanized monoclonal antibody against the envelope protein of Multiple Sclerosis-associated endogenous retrovirus: a first-in-humans randomized clinical study.
Human endogenous retrovirus (HERV) genes represent about 8% of the human genome. A member of the HERV family W, the Multiple Sclerosis-Associated Retrovirus (MSRV) gene, encodes an envelope protein (Env), which can activate a proinflammatory and autoimmune cascade through its interaction with Toll-like receptor 4. Due to its proinflammatory property and an inhibitory effect on oligodendrocyte precursor cell differentiation, the MSRV-Env protein could play a crucial role in the pathogeny of multiple sclerosis. GNbAC1 is a humanized monoclonal antibody of the immunoglobulin G4 type, which is directed against MSRV-Env. After validation of the MSRV-Env as a therapeutic target in preclinical experimental models, a clinical development program was initiated.
In these healthy male subjects, the safety and pharmacokinetic profiles of GNbAC1 appeared favorable. These findings are expected to allow for the launch of a Phase II development program for this innovative therapeutic approach in patients with multiple sclerosis. ClinicalTrials.gov identifier: NCT01699555.
However, rather than injecting antibodies to gobble up the viral envelope, given the real and theoretical problems with monoclonal antibodies, it would be better to keep Env from being produced in the first place. Maybe a protease inhibitor is the missing link. AIDS drugs didn’t work well until they had PI’s. Dr. Snyderman’s data suggests this was the case for him. I am happy to report that he remains stable at 32 months. Does a response to a PI imply exogenous virus? How far does a HERV have to get in its reproductive cycle before a PI would do some good? SFFV is a defective virus with a pathogenic envelope. If MSRV produces variable particles, some of which appear complete on EM, is it ever infectious?
Reading about MS, thinking about my own clinical presentation and putting it together with everything we have learned since XMRV entered our lives, ME/CFS may exist on a spectrum with MS, in the same way that Aspergers Syndrome is part of the autistic spectrum. Certainly, we are a variation on a theme. I have called it MS light before and I think it is a good working hypothesis for now. Up To Date’s summary on MS is here. Note the many similarities, genetics, epidemiology (including cluster outbreaks), possible problems with the Hepatitis B vaccine. It seems to me our best hope post XMRV is to ride on the coattails of MS, even though it is pathetic that we need to, given that there are at least three times as many of us.
I’m getting lots of questions about what I think of the paper published by De Meirlier et al. Plasmacytoid dendritic cells in the duodenum of individuals diagnosed with myalgic encephalomyelitis are uniquely immunoreactive to antibodies to human endogenous retroviral proteins. I am not going to evoke all the reasons why I might have a problem with this paper, whatever it says. I have moved on. Much of it is documented elsewhere on this blog.
Taking the paper at face value, problems with it are the tiny sample size, from patients that I hope had very serious GI complaints, compared to the patient population as a whole, since, presumably, they warranted a duodenal biopsy. I would like to take this opportunity to emphasize that I am completely opposed to taking any risk of harming fragile patients with unnecessary procedures in order to study the disease. There is no reason to do duodenal biopsies on garden variety ME patients, so the patients in this study should have had significant inflammatory bowel disease, not just IBS. The procedure carries a significant risk. A duodenal punch biopsy can result in death. There is lots of tissue to study without resorting to that. Fresh tissue is harvested all the time for other reasons, there is lots of material to autopsy and lots of specimens in paraffin, which is what was used in this study. My small intestine in paraffin is stored down the street at the local hospital. And plasmacytoid dendritic cells can be harvested from peripheral blood.
The simplest explanation for the findings in this paper is that there was a range of proteins consistent with a generalized activation of HERVs. Many things can transactivate HERVs including recombination events and exposure to exogenous retroviruses. Perhaps they didn’t name the HERV because they were all transactivated? This is what you might expect in someone with inflammatory bowel disease. We have no idea whether these people had a neuroimmune disease or not. The fact that they had a range of symptoms that would qualify for a clasification of CFS is neither here nor there. Endogenous retrovirus-K promoter: a landing strip for inflammatory transcription factors?
There are quite a few papers worth reading in the references, but they missed one: Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells.
I hope they are right. It would set us on a path to catch us up to MS, where we belong. However, the paper is so vague. Antibodies to proteins expressed by a generic HERV. This negative paper was also just published: Human Endogenous Retrovirus-K18 Superantigen Expression and Human Herpesvirus-6 and Human Herpesvirus-7 Viral Loads in Chronic Fatigue Patients. It is good news for us that this avenue of research is being pursued.
I expect the De Meirleir paper to get shot down or be ignored completely. The scientific world will probably only read it for laughs, considering the source. They didn’t find a “real” virus this time, so nobody needs to spend millions of dollars to prove it wrong. MSRV was ignored for decades, even though it is associated with a more sympathetic disease than ME/CFS. Progress with it has been glacial, revealing the non-urgent, almost lackadaisacal attitude of the biomedical world towards activated HERVs, even one that was shown to produce viral particles over 20 years ago. In any case, infectious or not, there is increasing agreement that HERV W is associated with MS and can transcribe an Env protein which is neuropathogenic.
And another related illness: HERVs expression in Autism Spectrum Disorders.
I am particularly happy to report that my friend Dr. Mikovits is doing well through it all. She has received many letters of support and asked me to let the community know that she is fine and excited about the future. She is consulting with respect to drugs and diagnostics. She continues to lecture. Currently, she is working on projects with Dr’s Ruscetti and Lipkin, and, in a translational capacity with several medical doctors, Eric Gordon, Chitra Bhakta, Derek Enlander, Paul Cheney, Michael Snyderman and myself.
This excerpt is from an email to me a couple of days ago when I asked her a few questions for this blog:
Planning for the April 25th FDA meeting…a two day meeting to get drug companies and clinical trials going..to avoid the failure of Hemispherx..we have a huge opportunity here..talk about that..tell the patient community I will go there and work to bring them the drugs that are out there as soon as possible..we as a community do not have to go back to basic research where we are decades away..we can translate what we know.. write about that …move forward..
My background is in antiviral drug mechanisms and epigenetic drug development..I am going back to my roots to focus on drug development in infectious/ inflammatory disease…I can now apply my expertise and extensive network to ME/CFS..
Dr. Lipkin said this about her in Nature, only a few months ago:
I feel very badly for Mikovits, [her co-author] Ruscetti and Harvey Alter [a hematologist at the NIH Clinical Center in Bethesda, Maryland, who led one of the CFS studies]. Mikovits in particular — she has lost everything. She can be wrong but she’s not a criminal. She has been honest in a respectful, forceful way and said that we have to conclude that we were wrong. You can imagine how difficult it must be, and I think she should be applauded. Lots of people wouldn’t have the balls to do that. She has come across as a scientist who really believes in the importance of truth.
Dr. Judy has come a long way since then, pulling herself up by her own bootstraps. I am in awe of her resilience. Handed lemons, she is making excellent lemonade. Stay tuned.
Today’s song: Titanium by David Guetta